AstraZeneca withdraws COVID-19 vaccine worldwide, cites commercial reasons
First Published: 8th May, 2024 9:52 IST
AstraZeneca has announced that the vaccine was being removed from markets for commercial reasons.
The pharmaceutical giant AstraZeneca‘s COVID-19 vaccine is being withdrawn worldwide after the company acknowledged for the first time in court documents that it can cause a rare and dangerous side effect, according to a report in the British Newspaper ‘The Telegraph’.
AstraZeneca has announced that the vaccine was being removed from markets for commercial reasons. It further said that the vaccine was no longer being made or supplied, having been superseded by updated vaccines that fight new variants.
The application to withdraw the vaccine was made on March 5 and came into effect on May 7. The vaccine can be no longer used in the European Union following the company’s decision to withdraw its “marketing authorisation.”
Similar applications will be submitted in the UK and other nations in the coming months that have given a go-ahead to the vaccine, known as Vaxzevria.
In recent months, Vaxzevria has come under scrutiny over a very rare side effect, which causes blood clots and low blood platelet counts. In court documents, AstraZeneca in the High Court in February admitted that the vaccine “can, in very rare cases, cause TTS“.
TTS which stands for Thrombosis with Thrombocytopenia Syndrome has been associated with at least 81 deaths in the UK and hundreds of serious injuries. More than 50 alleged victims and grieving relatives have filed a lawsuit against AstraZeneca in a High Court case.
AstraZeneca has insisted that the decision to withdraw the vaccine is not related to the case or admission that it can cause TTS and termed the timing a pure coincidence, according to The Telegraph report.
“We are incredibly proud of the role Vaxzevria played in ending the global pandemic. According to independent estimates, over 6.5 million lives were saved in the first year of use alone and over three billion doses were supplied globally. Our efforts have been recognised by governments around the world and are widely regarded as being a critical component of ending the global pandemic,” The Telegraph quoted AstraZeneca as saying.
In a statement, the pharmaceutical giant said, “As multiple, variant Covid-19 vaccines have since been developed, there is a surplus of available updated vaccines”, adding that it has led to a decline in demand for Vaxzevria, which is no longer being manufactured or supplied, The Telegraph reported.
It further announced its decision to initiate the withdrawal of the marketing authorisations for Vaxzevria within Europe. The company said, “We will now work with regulators and our partners to align on a clear path forward to conclude this chapter and significant contribution to the Covid-19 pandemic.”
Last week, AstraZeneca reiterated its commitment to patient safety while emphasising the vaccine’s overall safety profile.
An AstraZeneca spokesperson had stated, “Our sympathy goes out to anyone who has lost loved ones or reported health problems. Patient safety is our highest priority, and regulatory authorities have clear and stringent standards to ensure the safe use of all medicines, including vaccines.”
This comes in the wake of a recent admission by AstraZeneca, the pharmaceutical company, that its Covid vaccine Covishield and Vaxzevria “can, in very rare cases, cause Thrombosis Thrombocytopenia Syndrome (TTS).”
Despite these rare occurrences, the pharmaceutical company maintained that extensive clinical trial data and real-world evidence consistently support the vaccine’s safety and efficacy. Regulatory agencies worldwide continue to assert that the benefits of vaccination outweigh the risks of such extremely rare side effects. (ANI)
COMMENTS

TOPMOST STORY NOW
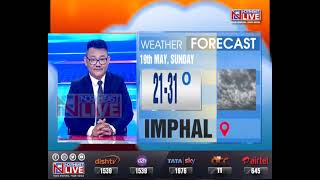
Live #NEWeatherUpdate: IMD predicts rainfall in 7 NE states in next 5 days
19th May 2024
Live CAA will not be implemented in areas under 6th Schedule in Northeast: Pradyot Debbarma
19th May 2024
Live Tripura: Notorious human trafficker Hannan Miah wanted by NIA arrested
19th May 2024
Live 65 people from the Bawm Tribe in Bangladesh enter Mizoram
19th May 2024WE RECOMMEND

IMD issues red alert for Delhi; temperature likely to touch 44 degrees
Senior IMD scientist Naresh Kumar said that the current situation will prevail for the next week.
19th May 2024
Mukesh Ambani to host Anant-Radhika’s second pre-wedding function on a grand cruise ship: Check details
The Ambani family will reportedly host the second pre-wedding function on a cruise ship off the coast of South France.
19th May 2024
Lok Sabha elections: Heavy security checks in Mumbai ahead of fifth phase of voting
Security forces have intensified vigil in Mumbai, a day before the metropolitan city is set to go into polls in the fifth phase of the ongoing Lok Sabha elections on Monday (May 20). The city remains under tight security cover as large-scale spot checks are being carried out by stationary and mobile teams. The city remains under […]
19th May 2024
Bihar: Shahi litchi of Muzaffarpur to remain costly due to heatwave spells
The heatwave conditions swept across various districts of Bihar.
19th May 2024
“Swati Maliwal ka Sach”: AAP attacks Maliwal after purported clip from day of “assault” goes viral
In a post on social media platform X, AAP stated, "Swati Maliwal ka sach (Swati Maliwal's truth)." The post refers to a news story with a purported video clip of the Rajya Sabha MP and security personnel at CM Kejriwal's house on May 13. Delhi Police say that the video is yet to be authenticated by them.
17th May 2024

